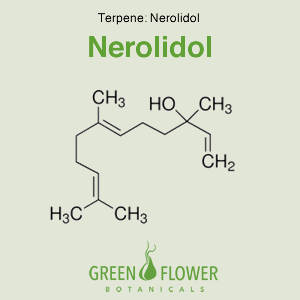
Nerolidol – Terpene
Description:
EFFECTS: Antiprotozoan, Topical Drug Enhancer.
Strains containing Nerolidol include:
Nerolidol, also known as peruviol, is a naturally occurring sesquiterpene found in the essential oils of many types of plants and flowers. There are two isomers of nerolidol, cis and trans, which differ in the geometry about the central double bond. Nerolidol smells similar to fresh bark. The terpene has been alluded to be a toxin against harmful protozoa like malaria and leishmaniasis. Furthermore, nerolidol is effective in delivering drugs through the skin.
⇩ View A – Z Index of Terpenes ⇩
Effects
Antiprotozoan
Topical Drug Enhancer
Research
Topical Drug Enhancer
Nerolidol has been shown to help the passive skin penetration enhancement, helping a model hydrophobic permeant increase its absorption into skin by 20 fold. There were also significantly longer durations of action and did not wash off easily.
Cornwell PA, Barry BW (1994). Sesquiterpene components of
volatile oils as skin penetration enhancers for the hydrophilic
permeant 5-fluorouracil. J Pharm Pharmacol 46: 261–269.
Antiprotozoan
The research on nerolidol has concluded it to be a potent malaria drug. 100 microg/mL of nerolidol caused 100% of growth inhibition of young trophozoite to schizont stage after 48 hours of exposure. Nerolidol had an inhibitory effect on the biosynthesis of the isoprenic and dolichol side chains of the benzoquinon ring of ubiquinones in the organism. Thus the expression of p21 ras protein was significantly decreased.
Lopes NP, Kato MJ, Andrade EH, Maia JG, Yoshida M, Planchart AR
et al. (1999). Antimalarial use of volatile oil from leaves of Virola
surinamensis (Rol.) Warb. by Waiapi Amazon Indians.
J Ethnopharmacol 67: 313–319.
Nerolidol has been proven to be effective with Leishmaniasis as well. 57-85 uM was proven to be effective as a 50% growth inhibitory concentration in a broad range of Leishmania species.100uM resulted in 95% reduction in infection rates of macrophages. The compound inhibits the mevalonate pathway. The compound inhibits isoprenoid synthesis by reducing the incorporation of mevalonic acid or acetic acid precursors into dolichol, ergosterol, and ubiquinone. This compound’s effect can be attributed to the blockage of an early step in the mevalonate pathway.
Arruda DC, D’Alexandri FL, Katzin AM, Uliana SR (2005).
Antileishmanial activity of the terpene nerolidol. Antimicrob
Agents Chemother 49: 1679–1687.
CBD Oils Containing Nerolidol

Alpha Bisabolol
EFFECTS: Analgesic, Anti-cancer, Anti-inflammatory, Antifibrosis, Antifungal, Antocoagulant, Drug Potentiator.
Learn about Alpha Bisabolol »

Alpha Pinene
EFFECTS: Anti-bacterial, Anti-cancer, Anti-inflammatory, Bronchodilator, Memory Enhancer.
Learn about Alpha Pinene »
Beta Caryophyllene
EFFECTS: Analgesic, Anti-inflammatory, Antioxidant, Gastric-protective.
Learn about Beta Caryophyllene »
Beta Pinene
EFFECTS: Expectorant, Bronchodilator, Anti-inflammatory, Antiseptic.
Learn about Beta Pinene »
Borneol
EFFECTS: Analgesic, Anti-cancer, Anti-inflammatory, Antifibrosis, Antifungal, Antocoagulant, Drug Potentiator.
Learn about Borneol »
Camphene
EFFECTS: Hypolipidemic, Anti-bacterial, Antifungal.
Learn about Camphene »
Caryophyllene Oxide
EFFECTS: Antifungal, Antocoagulant.
Learn about Caryophyllene Oxide »
Eucalyptol
EFFECTS: Anti-Alzheimer's, Anti-Asthma, Anti-bacterial, Anti-cancer, Anti-inflammatory, Antioxidant.
Learn about Eucalyptol »
Fenchol
EFFECTS: Antioxidant, Antimicrobial, Antifungal.
Learn about Fenchol »

Humulene
EFFECTS: Anti-bacterial, Anti-cancer, Anti-inflammatory.
Learn about Humulene »

Limonene
EFFECTS: Anti-anxiety, Anti-cancer, Anti-inflammatory, Antidepressant.
Learn about Limonene »

Linalool
EFFECTS: Analgesic, Anti-anxiety, Anti-inflammatory, Anticonvulsant, Sedative.
Learn about Linalool »

Myrcene
EFFECTS: Analgesic, Antioxidant, Sedative.
Learn about Myrcene »

Nerolidol
EFFECTS: Antiprotozoan, Topical Drug Enhancer.
Learn about Nerolidol »
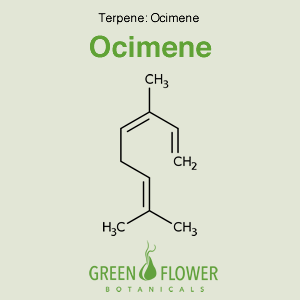
Ocimene
EFFECTS: Anti-inflammatory, Antifungal, Antiviral.
Learn about Ocimene »
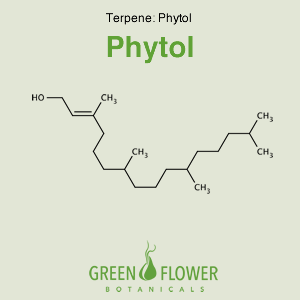
Phytol
EFFECTS: Antischistosomal, Anti-cancer, Hypolipidemic, Antidiabetic.
Learn about Phytol »
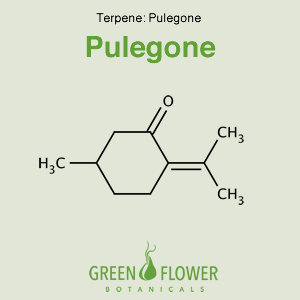
Pulegone
EFFECTS: Expectorant.
Learn about Pulegone »
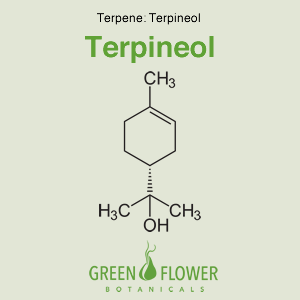
Terpineol
EFFECTS: Antibacterial, Antioxidant, Anti-cancer, Sedative, Anti-inflammatory.
Learn about Terpineol »

Terpinolene
EFFECTS: Anti-bacterial, Anti-cancer, Antioxidant, Sedative.
Learn about Terpinolene »
Valencene
EFFECTS: Anti-inflammatory.
Learn about Valencene »
